Comment:
This study is a critical piece of evidence that directly challenges a long-standing piece of medical dogma. For decades, we have routinely advised patients to stop fish oil supplements before surgery, fearing an increased risk of bleeding. This was solely based on hypothesis.
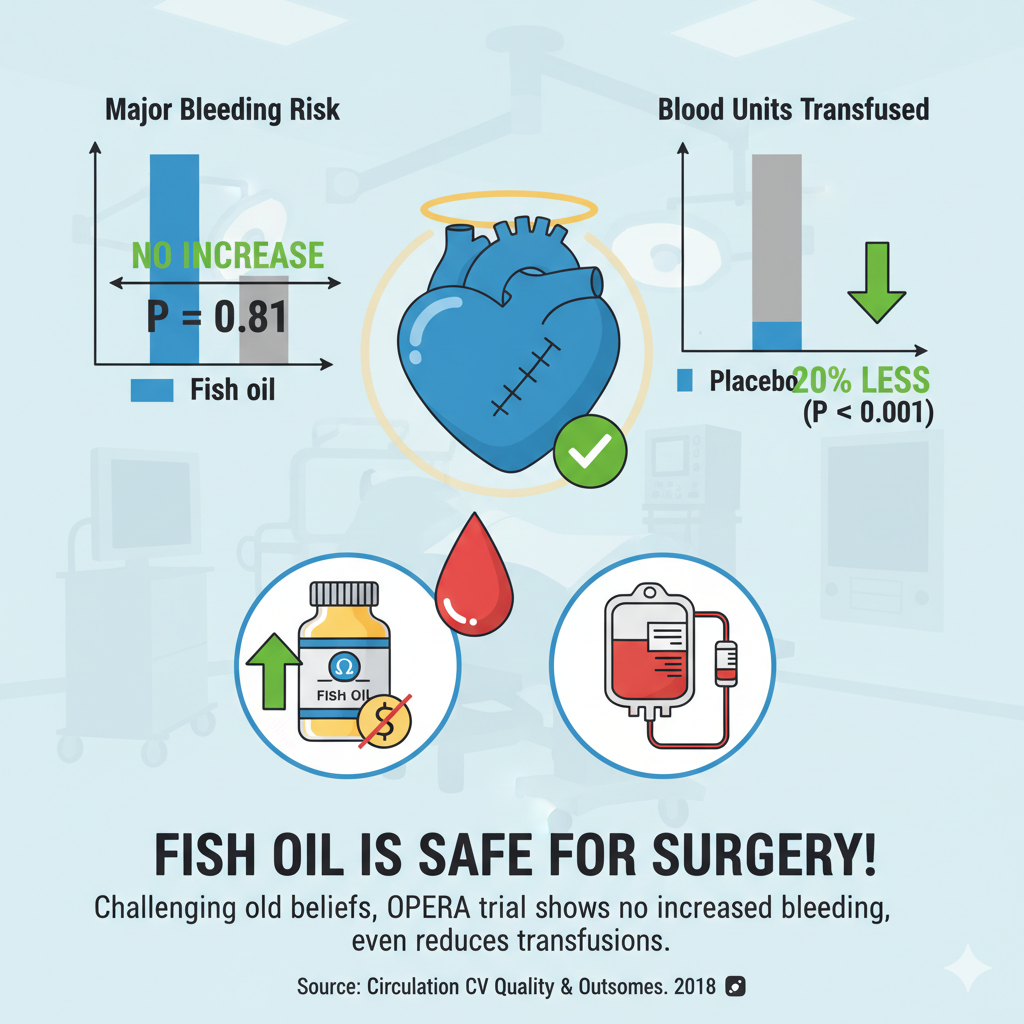
This robust analysis of the OPERA trial not only debunks that misconception—showing no increase in major bleeding—but it also reveals a surprising potential benefit. The association of fish oil with a significant reduction in the need for blood transfusions is a remarkable finding.
Furthermore, the biomarker data showing that patients with the highest omega-3 levels had the lowest bleeding risk is compelling. So not only is it incorrect that fish oils had to be stopped, it is actually causing harm.
Summary:
Clinical Bottom Line
This secondary analysis of the OPERA trial provides robust evidence that perioperative fish oil supplementation is safe for patients undergoing cardiac surgery. The study found that fish oil did not increase the risk of major bleeding compared to placebo.
Unexpectedly, fish oil was associated with a statistically significant reduction in the number of blood transfusions required. Furthermore, a biomarker analysis in a subset of patients found that higher achieved blood levels of omega-3 fatty acids were associated with a lower risk of bleeding. These findings directly challenge the common clinical practice of advising patients to stop fish oil supplements before surgery due to theoretical bleeding concerns.
Results in Context
Primary Outcome
This analysis focused on major perioperative bleeding as defined by the Bleeding Academic Research Consortium (BARC) type 4 or 5 criteria. This is a composite endpoint including severe outcomes like fatal bleeding, reoperation for bleeding, or requiring a large-volume transfusion (≥ 5 units).
-
Result: There was no significant difference in the risk of major bleeding between the groups.
-
Statistics: The primary outcome occurred in 5.51% of the fish oil group versus 6.61% of the placebo group. The odds ratio was 0.81 (95% CI, 0.53-1.24). This means the odds of a major bleed were 19% lower in the fish oil group, but this result was not statistically significant (as the 95% confidence interval crosses 1.0), indicating no detectable difference in risk.
Key Secondary Outcomes
-
Other Bleeding Definitions: The finding of no increased bleeding risk was consistent across multiple other standardized definitions, including TIMI major, TIMI minor, and International Society on Thrombosis and Hemostasis (ISTH) surgical bleeding.
-
Blood Transfusions: The fish oil group required significantly fewer total units of blood transfused compared to the placebo group (mean 1.61 units vs. 1.92 units, P < 0.001). This reduction was seen for transfusions both during (P = 0.002) and after (P = 0.006) surgery.
-
Biomarker Analysis: In a subset of 564 patients, higher achieved plasma levels of omega-3s (measured on the morning of surgery) were significantly associated with a lower risk of BARC bleeding. Compared to patients with the lowest levels (first quartile), those in the third quartile had a 70% lower risk (OR 0.30) and those in the fourth quartile had a 64% lower risk (OR 0.36).
Harms and Adverse Events
The primary harm investigated (perioperative bleeding) was not increased by fish oil supplementation.
Minor, non-serious adverse events were more common in the fish oil group, including gastrointestinal upset, burping, and a fish oil taste. Other serious adverse events were reported as generally similar between the two groups.
Assertive Critical Appraisal
Risk of Bias (RoB 2 Framework)
Overall judgment: Some concerns.
This study is a secondary analysis of the OPERA trial, which was originally designed to test fish oil for the prevention of postoperative atrial fibrillation.
-
The randomization, robust blinding (patients, clinicians, and investigators), and use of standardized, objective bleeding definitions mean the measurement of these safety outcomes has a low risk of bias.
-
The fact that bleeding outcomes were pre-specified safety endpoints of the original trial adds credibility to the main finding of “no harm”.
-
However, the trial was not originally designed or powered to test the hypothesis that fish oil could reduce bleeding. Therefore, the novel findings of reduced transfusions and the inverse association between omega-3 levels and bleeding risk should be considered exploratory and hypothesis-generating.
Subgroup Analyses
The authors performed exploratory subgroup analyses and noted the reduction in blood transfusions appeared more pronounced in patients undergoing valve surgery or minithoracotomy. As these analyses were exploratory, the authors correctly advise they “should be viewed with caution”.
Composite Endpoints
The primary BARC endpoint is a composite of several severe outcomes (e.g., fatal bleeding, reoperation, large transfusion). These components are all clinically important and of similar severity, making this a reasonable composite endpoint for major bleeding.
Reporting Quality Assessment (CONSORT)
The paper’s reporting quality is high. It clearly describes the methods for randomization (computer-generated), allocation concealment (central coordinating center), and blinding (identical capsules, all parties blinded). Participant numbers are clearly stated.
Applicability
The findings of this study are highly applicable to clinical practice. The trial included a broad, multinational population (US, Italy, Argentina) undergoing common high-risk cardiac surgeries (CABG and valve surgery). The results provide strong evidence to reconsider the widespread, but largely theoretical, recommendation to stop fish oil supplementation before cardiac surgery. The authors note that generalizability to other types of non-cardiac surgery is not established by this trial.
Research Objective
The objective of this secondary analysis was to evaluate the effect of perioperative fish oil supplementation on a range of standardized perioperative bleeding outcomes, using data from the OPERA randomized trial.
Study Design
-
Design: This is a secondary analysis of a multinational, double-blind, placebo-controlled, randomized trial (OPERA).
-
Allocation: 1516 patients were randomized 1:1.
-
Intervention: Fish oil (465 mg EPA + 375 mg DHA per 1-g capsule). Patients received a loading dose of 8-10 g over 2-5 days before surgery, followed by 2 g/day postoperatively.
-
Control: Matched placebo (olive oil) capsules.
-
Blinding: All investigators, patients, and providers were blinded to treatment assignment.
Setting and Participants
-
Setting: 28 medical centers in the United States, Italy, and Argentina.
-
Participants: 1516 patients (≥ 18 years) scheduled to undergo cardiac surgery (CABG, valve surgery, or other).
-
Characteristics: The mean age was 63 years, and 72% were men. 52% had planned CABG and 50% had planned valve surgery.
-
Key Exclusions: Regular use of fish oil (≥ 3 d/wk) within the prior 4 weeks or a known allergy to fish or olive oil.
Bibliographic Data
-
Title: Fish Oil and Perioperative Bleeding: Insights From the OPERA Randomized Trial
-
Authors: Emmanuel Akintoye, Prince Sethi, William S. Harris, Paul A. Thompson, Roberto Marchioli, Luigi Tavazzi, Roberto Latini, Mias Pretorius, Nancy J. Brown, Peter Libby, Dariush Mozaffarian
-
Journal: Circulation: Cardiovascular Quality and Outcomes
-
Year: 2018
-
DOI: 10.1161/CIRCOUTCOMES.118.004584
Original Article:
Full text: PubMed Central
