Comment:
A critical takeaway from this real-world data is the apparent lack of protection for oncology patients. The finding for the immunocompromised subgroup—which includes many patients with active cancer or on immunosuppressive therapy—is stark.
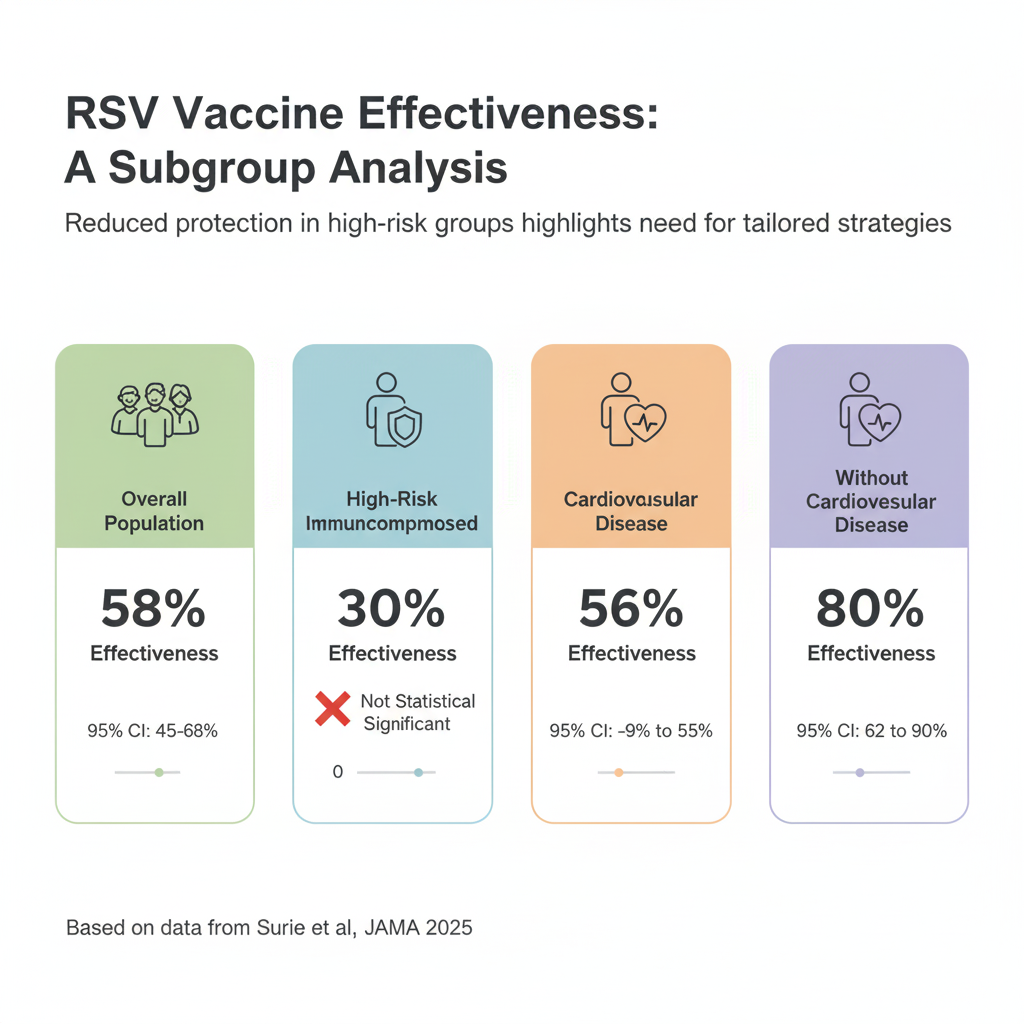
A vaccine effectiveness of 30% that is not statistically significant (95% CI, -9% to 55%) means we cannot conclude there is any benefit for this group.
It is effective for patients with cardiovascular disease, but significantly less than for those without.
Summary:
Clinical Bottom Line
This large, real-world study shows a strong association between a single RSV vaccine dose and a 58% reduction in RSV-associated hospitalization among adults 60 and older over two seasons. As an observational study, this provides valuable “real-world evidence” but cannot definitively prove causation due to the potential for unmeasured confounding variables.
However critically, the vaccine’s effectiveness was significantly lower in patients with cardiovascular disease (56% VE) compared to those without these conditions.
For immunocompromised patients the 30% effectiveness was not statistically significant, suggesting the vaccine offers no protection for this specific group.
Results in Context
Main Results
-
Overall Effectiveness: The primary finding was a vaccine effectiveness (VE) of 58% (95% CI, 45%-68%) against RSV-associated hospitalization across two seasons.
-
Waning Effectiveness: The study observed a non-statistically significant trend toward waning protection. Effectiveness was 69% (95% CI, 52%-81%) for vaccination in the same season as illness, compared to 48% (95% CI, 27%-63%) for vaccination in the prior season ($P=.06$).
-
Effectiveness in High-Risk Subgroups:
-
Immunocompromised: VE was 30% (95% CI, -9% to 55%). This was significantly lower than the 67% (95% CI, 53%-77%) VE seen in immunocompetent adults (P=.02).
-
Cardiovascular Disease: VE was 56% (95% CI, 32%-72%). This was also significantly lower than the 80% (95% CI, 62%-90%) VE in adults without this condition (P=.03).
-
Definitions
-
Vaccine Effectiveness (VE): This was calculated as (1 – adjusted odds ratio) x 100%. A VE of 58% means there was a 58% reduction in the odds of being hospitalized for RSV among the vaccinated group compared to the unvaccinated group, after adjusting for confounding factors.
-
Confidence Interval (CI): The 95% CI for the immunocompromised subgroup was -9% to 55%. Because this interval crosses zero, the 30% point estimate is not statistically significant. This means the data are consistent with a range of effects, from the vaccine having no benefit (or even slight harm) to a moderate 55% benefit.
-
Moderate or severe immunocompromise was defined as having any of the following:
-
Active solid tumor or hematologic malignancy (defined as a new diagnosis or treatment for the malignancy within the previous 6 months)
-
Solid organ transplant
-
Hematopoietic cell transplant
-
HIV infection
-
Primary immunodeficiency
-
Use of immunosuppressive medication in the past 30 days
-
Other conditions that cause moderate or severe immunosuppression
-
Participants
-
A total of 6958 adults aged 60+ hospitalized with acute respiratory illness were included in the analysis.
-
This included 821 (11.8%) RSV cases (tested positive for RSV only) and 6137 (88.2%) controls (tested negative for RSV, SARS-CoV-2, and influenza).
-
A significant portion, 1829 adults (26.3%), were classified as moderately or severely immunocompromised.
-
Overall vaccination uptake was low: 63 cases (7.7%) and 966 controls (15.7%) were vaccinated.
Assertive Critical Appraisal
Limitations & Bias (STROBE Framework)
This study’s primary methodological challenge is confounding, which is inherent to all observational research.
-
Confounding by Indication/Health Behaviors: The authors correctly identify that the low vaccination rate in the control group (15.7%) suggests that “early adopters of new vaccines may differ from the general vaccine-eligible population”. This can lead to residual confounding, where unmeasured factors (like mask-wearing, socioeconomic status, or general health-seeking behaviors) are the true cause of the observed association, rather than the vaccine itself.
-
Insufficient Subgroup Power: The authors explicitly state that the “sample size was insufficient to precisely estimate VE by time since vaccination in certain high-risk subgroups (eg, individuals with immunocompromise)”. This is a critical admission and is the statistical reason why the confidence interval for the immunocompromised group was too wide to draw a firm conclusion.
-
Unaccounted Variables: The analysis did not account for protection from a “recent prior RSV infection,” which could also confound the results.
Reporting Quality Assessment (STROBE)
This study adheres to the STROBE reporting guideline. As required for a high-quality observational study, the authors clearly describe their efforts to address potential sources of confounding. They used multivariable logistic regression adjusted for key variables: age, sex, race and ethnicity, geographic region, and calendar month/year. The test-negative design itself is a key strength used to mitigate confounding from health-seeking behaviors, as both cases and controls were patients who sought care for an acute respiratory illness.
Reporting Quality Assessment (RECORD) for RWE Studies
This study uses real-world data from the IVY Network. Its reporting quality is high. The authors clearly describe:
-
Data Sources: Data were gathered from “patient interview and electronic health records”. Vaccination status was verified using a hierarchy of “electronic health records, state or city vaccine registries, and self-report”.
-
Participant Selection: The process is detailed, enrolling patients hospitalized for acute respiratory illness at 26 hospitals. A flow diagram (Figure 1) details the exclusion of over 6,000 patients from the initial 13,159 enrolled.
-
Variable Definitions: All key variables (case, control, vaccinated, and immunocompromised) are explicitly defined.
Applicability
The findings are highly applicable to a general clinical practice. The study was conducted in a large, diverse US population across 26 hospitals in 20 states. Most importantly, it included a large cohort of high-risk patients (26.3% immunocompromised) who were actively excluded from the original prelicensure vaccine trials. This study provides the exact real-world data needed to guide vaccination discussions with these more complex patients.
Research Objective
To evaluate the effectiveness of the RSV vaccine against RSV-associated hospitalization among adults aged 60 years or older during two RSV seasons.
Study Design
This was a test-negative, case-control study conducted prospectively through the Investigating Respiratory Viruses in the Acutely Ill (IVY) Network.
-
Cases: 821 adults (60+) hospitalized with acute respiratory illness who tested positive for RSV only.
-
Controls: 6137 adults (60+) from the same hospitals who tested negative for RSV, SARS-CoV-2, and influenza.
-
Analysis: The odds of prior RSV vaccination were compared between cases and controls using multivariable logistic regression to calculate an adjusted odds ratio, which was then used to estimate vaccine effectiveness.
Setting and Participants
-
Setting: 26 hospitals across 20 US states.
-
Dates: Participants were enrolled during two RSV seasons: October 1, 2023, to March 31, 2024, and October 1, 2024, to April 30, 2025.
-
Eligibility: Adults aged 60 years or older hospitalized with an acute respiratory illness who had respiratory virus testing within 10 days of illness onset.
Bibliographic Data
-
Title: RSV Vaccine Effectiveness Against Hospitalization Among US Adults Aged 60 Years or Older During 2 Seasons
-
Authors: Diya Surie, MD; Wesley H. Self, MD, MPH; Katharine A. Yuengling, MPH; et al, for the Investigating Respiratory Viruses in the Acutely Ill (IVY) Network
-
Journal: JAMA
-
Year: 2025
-
DOI: 10.1001/jama.2025.15896
This AI-generated analysis is for informational and research purposes only and is not a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of a qualified health provider with any questions you may have regarding a medical condition.
Original Article:
Full text pdf: available here.
