Comment:
This is an important, large-scale population study, even with the weaknesses of the study design. An adjusted odds ratio of 1.50 for any cancer is a significant signal that cannot be easily dismissed, particularly given the strong associations noted for brain (AOR 1.90) and breast (AOR 1.24) cancers.
While the authors rightly point out the limitations—chiefly the unmeasured confounders like smoking and BMI—the robustness of this registry data (over 600,000 cases) suggests this isn’t just statistical noise. This study is valuable because it identifies a plausible risk that has, until now, been largely overlooked.
This research provides a valid reason for increased caution and until we have that clarifying data, this potential risk should be discussed with patients.
Summary:
Clinical Bottom Line
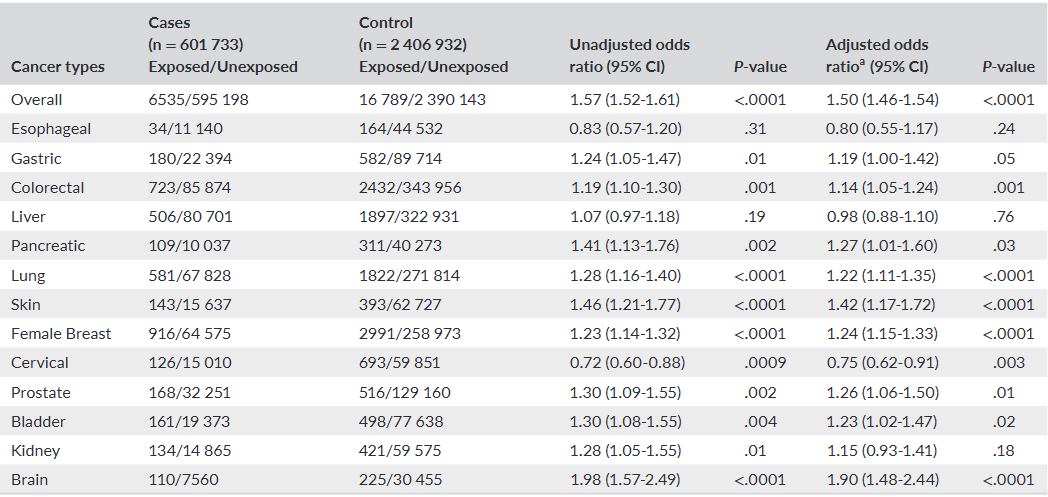
This large, registry-based case-control study found a statistically significant association between levothyroxine use and a 50% increased risk of any cancer, with particularly high associations for brain, skin, pancreatic, and breast cancers. However, this study cannot prove causation. The findings are significantly limited by the lack of data on major cancer risk factors like smoking, alcohol consumption, and Body Mass Index (BMI), which introduces a high risk of unmeasured confounding. The authors appropriately caution that these findings should be interpreted with caution and do not advise against the use of levothyroxine, which remains a highly effective therapy.
Results in Context
-
Main Results: After adjusting for available confounders, levothyroxine users had a 50% higher odds of having any cancer compared to non-users.
-
Overall Cancer: Adjusted Odds Ratio (AOR) 1.50 (95% CI: 1.46–1.54)
-
Brain Cancer: AOR 1.90 (95% CI: 1.48–2.44)
-
Skin Cancer: AOR 1.42 (95% CI: 1.17–1.72)
-
Pancreatic Cancer: AOR 1.27 (95% CI: 1.01–1.60)
-
Female Breast Cancer: AOR 1.24 (95% CI: 1.15–1.33)
-
Definitions: An Adjusted Odds Ratio (AOR) of 1.50 means that, after controlling for factors like age, sex, and certain comorbidities, the odds of being a levothyroxine user were 50% higher among patients with cancer (cases) compared to the matched patients without cancer (controls).
-
Other Findings:
-
The association with overall cancer risk was present for both short-term (60-364 days) and long-term (≥1 year) users.
-
Female patients had a relatively higher associated risk (AOR: 1.56) than male patients (AOR: 1.32).
-
Interestingly, levothyroxine use was associated with a decreased risk of cervical cancer (AOR: 0.75, 95% CI: 0.62–0.91).
-
-
Participants: The study included 601,733 cancer cases and 2,406,932 matched controls.
Assertive Critical Appraisal
-
Limitations & Bias (STROBE Framework):
-
Primary Flaw (Unmeasured Confounding): The most significant limitation, acknowledged by the authors, is that the database lacked information on critical cancer risk factors. There was no data on smoking status, alcohol consumption, or body mass index (BMI). These are all powerful, independent risk factors for many cancers. While the authors used a diagnosis of chronic obstructive pulmonary disease (COPD) as a surrogate for smoking, this is an imperfect measure that cannot capture the full confounding effect. It is impossible to know if the observed association is due to levothyroxine or these unmeasured lifestyle factors.
-
Detection Bias: Patients with hypothyroidism, who are prescribed levothyroxine, are likely to have more frequent contact with the healthcare system. This increased medical surveillance could lead to a higher rate of cancer detection (especially for less symptomatic cancers) compared to the general population controls, biasing the results away from the null.
-
Indication Bias / Confounding by Indication: The study links the drug (levothyroxine) to cancer, but it cannot separate this from the underlying disease (hypothyroidism). It is possible that the autoimmune or metabolic state of hypothyroidism itself, rather than its treatment, is associated with cancer risk.
-
Inability to Assess Overtreatment: The database could not distinguish between patients receiving appropriate hormone replacement and those who were overtreated (i.e., made hyperthyroid). It is biologically plausible that excessive thyroid hormone levels, which can increase oxidative stress, could be the source of the association, not appropriate replacement therapy.
-
-
Reporting Quality Assessment (RECORD Framework for RWE):
-
This study uses Real-World Data from a national health database. The authors’ reporting on their methods is generally strong.
-
They clearly describe the data source (Taiwan’s HWDC database), how participants were selected, and how the exposure (levothyroxine, ATC code H03AA01) and outcomes (cancer, ICD-9: 140-208) were defined.
-
The study includes a clear flowchart detailing the inclusion and exclusion criteria.
-
-
Applicability: The study was conducted exclusively in a Taiwanese population. These findings may not be generalizable to other populations with different races, ethnicities, or lifestyle risk factors. Given the significant limitations, these results do not support changing clinical practice or withholding levothyroxine from patients with hypothyroidism.
Research Objective
The study’s objective was to investigate the magnitude of the possible association between levothyroxine use and the risk of cancer at any site.
Study Design
-
This was a retrospective, population-based case-control study.
-
Cases were defined as all patients aged 20 years or older who had a first-time cancer diagnosis (ICD-9 codes 140-208) between 2001 and 2011.
-
Controls were selected from the same database. For each case, four individuals without a cancer diagnosis were matched based on age, sex, and the index date (the date of the case’s cancer diagnosis).
-
Exposure was defined as having been prescribed levothyroxine (ATC code H03AA01) for at least 2 months within the 3 years prior to the index date. This 3-year “lag” was intended to reduce reverse causation bias (i.e., prescribing the drug for early symptoms of an undetected canc1er).
Setting and Participants
-
Setting: The study used de-identified data from Taiwan’s Health and Welfare Data Science Center (HWDC) database, which covers over 99% of the Taiwanese population of 23 million.
-
Participants: The final analysis included 601,733 cancer cases and 2,406,932 matched controls. The mean age was 60.47 years, and 53.96% of participants in both groups were male.
Bibliographic Data
-
Title: Risk of cancer in long-term levothyroxine users: Retrospective population-based study.
-
Authors: Chieh-Chen Wu, Md. Mohaimenul Islam, Phung-Anh Nguyen, Tahmina Nasrin Poly, Ching-Huan Wang, Usman Iqbal, Yu-Chuan (Jack) Li, Hsuan-Chia Yang.
-
Journal: Cancer Science.
-
Year: 2021.
-
DOI: 10.1111/cas.14908.
Mandatory Disclaimer: This AI-generated analysis is for informational and research purposes only and is not a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of a qualified health provider with any questions you may have regarding a medical condition.
Original Article:
Full text pdf: Risk of cancer in long‐term levothyroxine users Retrospective population‐based study
Attribution-NonCommercial 4.0 International
CC BY-NC 4.0
Deed Canonical URL – https://creativecommons.org/licenses/by-nc/4.0/
