Comment:
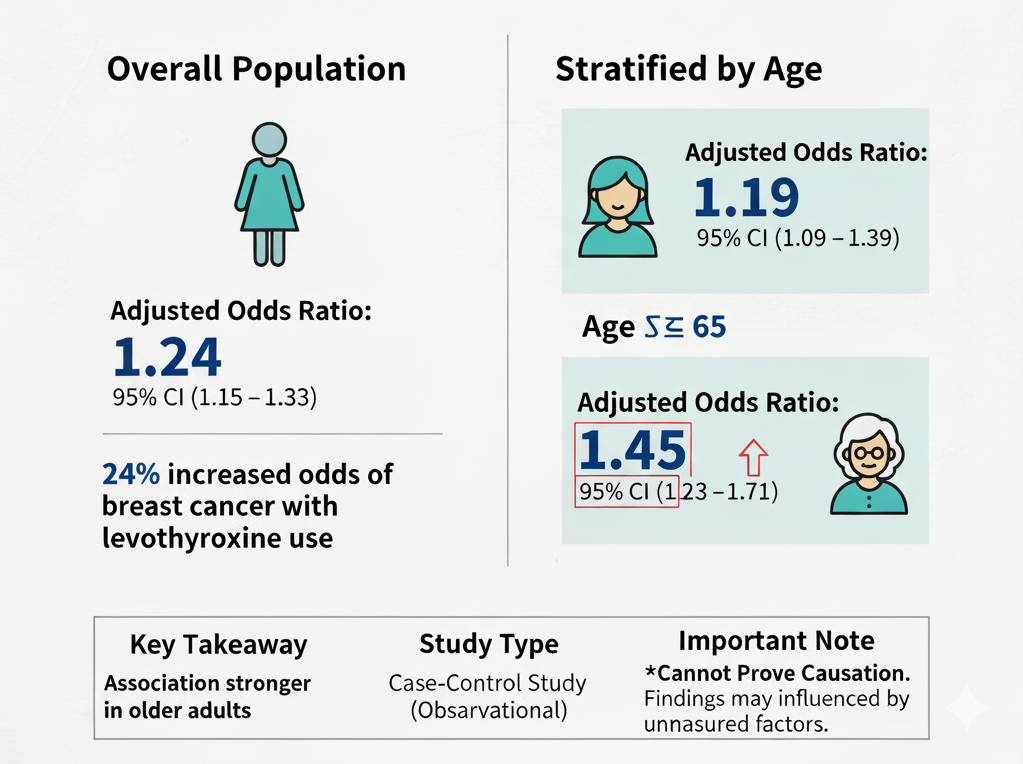
While this is an observational study and cannot prove causation, the findings are nonetheless concerning, especially as it’s not the only study showing an association of levothyroxine use and cancer. A nation-wide study identifying a 24% increase in the odds of breast cancer associated with levothyroxine use (AOR 1.24) warrants serious attention from the clinical community.
The association appears particularly robust in women aged 65 and older, who showed a 45% increase in odds (AOR 1.45). What makes this finding more troubling is that the risk was evident even in short-term users (60 days to 1 year), which challenges the assumption that only long-term, cumulative exposure might be problematic.
However, the study’s primary weakness—significant unmeasured confounding—is critical. The inability to adjust for key risk factors like BMI, family history, or the underlying severity of the thyroid dysfunction itself means we cannot be sure if we are seeing a true medication effect or an artifact of the underlying disease.
Ultimately, this study raises an important safety signal. It highlights the urgent need for further research, particularly prospective studies that can control for these key confounders, to clarify this potential and significant risk.
Summary:
Clinical Bottom Line
This large, population-based case-control study from Taiwan identifies a statistically significant association between levothyroxine use and a modest increase in the risk of breast cancer (Adjusted Odds Ratio 1.24). This association appeared stronger in patients aged 65 or older (AOR 1.45) and was present in both short-term (60 days to 1 year) and long-term (over 1 year) users.
However, as an observational study, these findings cannot prove causation and may be influenced by significant unmeasured confounding variables (like BMI, family history, and the underlying severity of thyroid disease), a major limitation the authors acknowledge. The authors state this is the first study to suggest this link and call for further research to confirm the finding.
Results in Context
Main Results
After adjusting for comorbidities and co-medications, the study found:
-
Overall Risk: Levothyroxine use was associated with a 24% increase in the odds of having breast cancer compared to non-users (Adjusted Odds Ratio [AOR] 1.24, 95% Confidence Interval [CI] 1.15–1.33).
-
Stratification by Age: The association was stronger in the older age group.
-
Age < 65 years: AOR 1.19 (95% CI 1.09–1.29).
-
Age ≥ 65 years: AOR 1.45 (95% CI 1.23–1.71).
-
-
Stratification by Duration of Use: A significant risk was seen in both short-term and long-term users compared to non-users.
-
60 days to 1 year: AOR 1.22 (95% CI 1.11–1.35).
-
Over 1 year: AOR 1.26 (95% CI 1.12–1.41).
-
Definitions
Case-Control Study: An observational study design that starts by identifying individuals with a specific outcome (the “cases” = breast cancer) and individuals without that outcome (the “controls”). It then looks back in time to compare how frequently the exposure (levothyroxine use) occurred in each group.
Adjusted Odds Ratio (AOR): A measure of association. In this study, an AOR of 1.24 means the odds of having used levothyroxine (for at least 2 months) were 24% higher among the breast cancer cases than in the matched controls, after statistically adjusting for differences in comorbidities and use of other medications.
Participants
The study identified 90,718 breast cancer patients from 2001-2011 in the Taiwanese national database. After applying exclusion criteria (e.g., excluding patients diagnosed from 2001-2003 and those under 20), the final cohort included 65,491 breast cancer cases. Each case was matched 1:4 with 261,964 controls.
Assertive Critical Appraisal
Limitations & Bias (STROBE Framework)
The primary limitation of any observational study is confounding—where an unmeasured factor is associated with both the exposure and the outcome, creating a spurious association.
-
Critical Unmeasured Confounders: The authors appropriately acknowledge that the database lacked information on several major potential confounders. This is the study’s most significant weakness. Missing data includes:
-
Key Breast Cancer Risk Factors: Body Mass Index (BMI), socioeconomic status, and family history of breast cancer.
-
Underlying Disease Severity: The study could not adjust for the reason levothyroxine was prescribed (e.g., hypothyroidism vs. goiter), the severity of the thyroid disease, or the presence of thyroid antibodies (like in Hashimoto’s disease). It is plausible that the underlying thyroid condition, not the medication, is linked to breast cancer risk.
-
Hormone Levels: The analysis lacked data on TSH, T3, or T4 levels.
-
-
Missing Clinical Detail: The database also lacked information on breast cancer histopathology, such as estrogen receptor (ER) or HER2 status. This prevents a more nuanced analysis of whether levothyroxine is associated with specific, hormonally-driven breast cancer subtypes.
-
Lack of Dose-Response: The authors note that a formal dose-response calculation (linking higher doses to higher risk) was not performed. The “duration” analysis showed a very similar risk for short-term and long-term users, which does not strongly support a cumulative dose effect.
Reporting Quality Assessment (RECORD) for RWE Studies
This study uses Real-World Data (RWE) from an insurance database. Its reporting quality against the RECORD guidelines is strong:
-
Data Source: Clearly identified as the Taiwan National Health Insurance Research Database (NHIRD).
-
Variable Definitions: The authors were transparent in how they defined:
-
Cases (Outcome): First-time breast cancer diagnosis (ICD-9-CM code 174), confirmed by linkage to the National Cancer Registry.
-
Exposure: Levothyroxine use was defined from electronic prescription records as continuous use for at least 2 months. “No users” were defined as those with < 2 months of use or no prescription.
-
Confounders: Comorbidities were identified by diagnostic codes and co-medications by prescription records.
-
Applicability
The study was conducted exclusively in the Taiwanese population. As the authors note, this association might vary in other populations or ethnic groups, limiting its generalizability.
Research Objective
To investigate whether the use of levothyroxine was associated with breast cancer risk.
Study Design
This was a nation-wide, population-based case-control study conducted using records from the Taiwan National Health Insurance Research Database (NHIRD). Cases consisted of patients aged 20 or older with a first-time breast cancer diagnosis between 2001 and 2011. Each case was matched 1:4 to four controls based on age, sex, and year/month of diagnosis (index date).
Setting and Participants
The study used the Taiwan National Health Insurance Research Database (NHIRD), which covers over 99% of the Taiwanese population. Cases were women aged 20 or older with a first-time breast cancer diagnosis (ICD-9-CM code 174) between 2004 and 2011. Patients diagnosed between 2001-2003 or those with a previous cancer diagnosis were excluded. The final analysis included 65,491 breast cancer cases and 261,964 matched controls.
Bibliographic Data
-
Title: Levothyroxine use and the risk of breast cancer: a nation-wide population-based case-control study
-
Authors: Chieh-Chen Wu, Ya-Yu Yu, Hsuan-Chia Yang, Phung Anh Nguyen, Tahmina Nasrin Poly, Md. Mohaimenul Islam, Usman Iqbal, Hafash Arshed Ali Khan, Yao-Chin Wang, Yung-Tzu Cheng, Yu-Chuan Li, Wen-Shan Jian
-
Journal: Archives of Gynecology and Obstetrics
-
Year: 2018
Original Article:
Original Article here
