Comment:
This is yet another high-quality, randomized controlled trial that undermines the long-standing bias against Desiccated Thyroid Extract (DTE). The overall finding is clear: for the average hypothyroid patient, DTE is non-inferior—meaning equivalent—to synthetic levothyroxine (LT4) when it comes to quality of life, memory, and depressive symptoms.
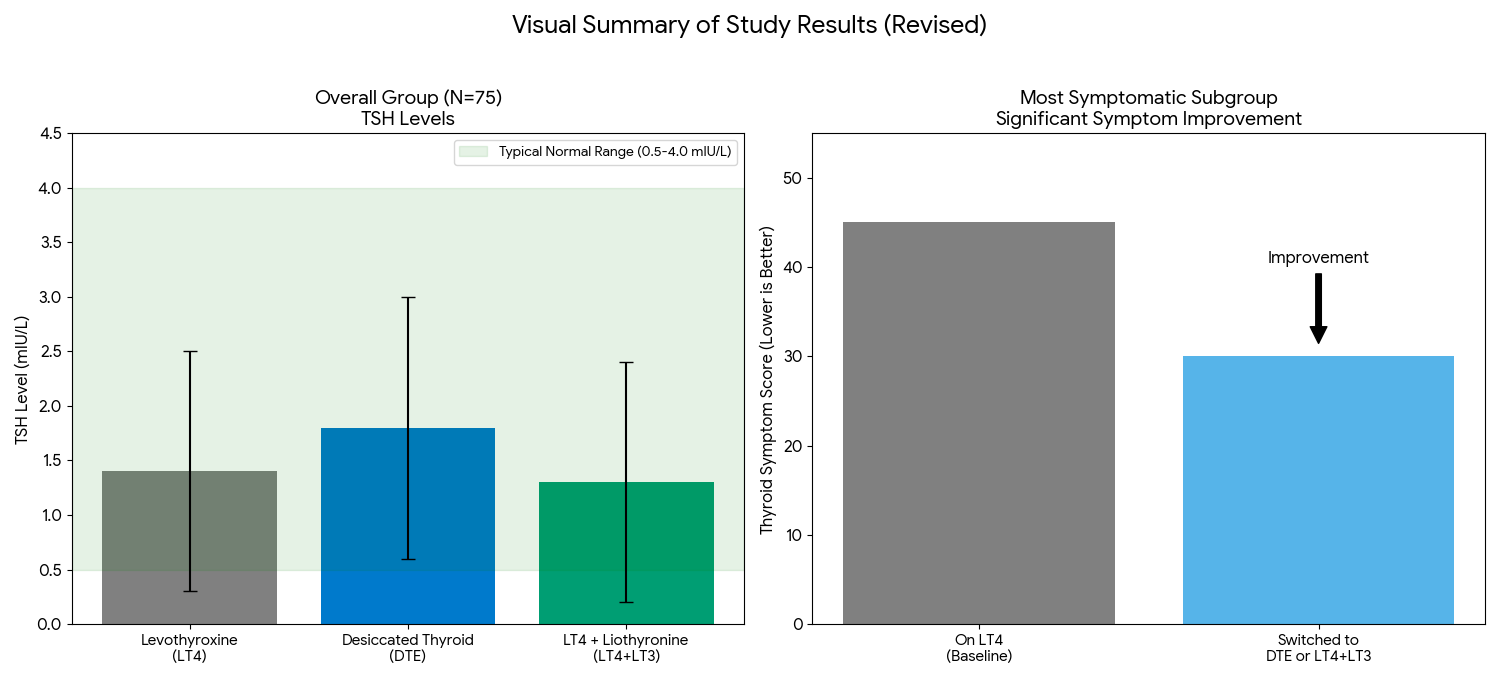
The most compelling takeaway (although a post hoc subgroup analysis) is that the group who needed it most—the one-third of patients who were still symptomatic on LT4 monotherapy—saw statistically significant symptom improvement when they switched to a T3-containing product, which includes DTE.
Summary:
🩺 Clinical Bottom Line
This study provides moderate evidence that for the average hypothyroid patient, there is no significant difference in symptoms, quality of life, memory, or depression between levothyroxine (LT4) monotherapy, desiccated thyroid extract (DTE), and combination LT4 + liothyronine (LT3).
However, the key finding is from a subgroup analysis: the one-third of patients who were most symptomatic while on LT4 monotherapy showed statistically significant improvements in symptoms, mood, and quality of life after switching to a T3-containing therapy (either DTE or LT4+LT3). While this subgroup analysis was pre-specified, it was not corrected for multiplicity, so this finding should be interpreted with caution as it could be due to chance.
📊 Results in Context
Primary Outcome
The trial evaluated four co-primary outcomes:
-
Thyroid Symptom Questionnaire (TSQ-36) score
-
General Health Questionnaire (GHQ-12) score
-
Beck Depression Inventory (BDI) score
-
Wechsler Memory Scale (WMS-IV) scores
In the main analysis of all 75 patients, there were no statistically significant differences found between the three treatment arms (LT4, DTE, and LT4+LT3) for any of these primary outcomes.
Key Secondary & Specialized Outcomes
-
Patient-Reported Outcomes (PROs): The study used several PRO tools as primary outcomes. While no differences were seen in the group as a whole, a key subgroup analysis focused on the 1/3 of patients who were most symptomatic while on LT4 (the “High” tercile). This subgroup showed significant improvements on the TSQ-36, GHQ-12, and BDI when they were switched to either LT4+LT3 or DTE.
-
Treatment Preference: In the overall group, there was no significant difference in treatment preference. However, in the subgroup of most symptomatic patients, preference “mass[ed] away from LT4,” with a strong preference for T3-containing therapies.
-
Biochemical Outcomes: While TSH remained within the normal range for all groups, it was slightly higher in the DTE group. Treatment with DTE or LT4+LT3 resulted in significantly higher fasting T3 levels and lower T4 levels compared to LT4 monotherapy.
Harms and Adverse Events
No serious adverse effects were reported, and no patients withdrew from the study due to side effects. All treatments were well-tolerated. There was a small, statistically significant increase in mean heart rate in the DTE group (73.7 bpm) compared to the LT4 group (71.5 bpm), but this was not associated with any adverse events.
🔬 Assertive Critical Appraisal
Risk of Bias (RoB 2 Framework)
The overall risk of bias is judged to have Some Concerns.
-
Strengths: The study was randomized and double-blind, using identical capsules prepared by a pharmacy to ensure allocation concealment and blinding of participants, investigators, and outcome assessors. The crossover design, where each patient served as their own control, is also a major strength.
-
Weaknesses: There is a significant reporting contradiction regarding study dropouts. The text states 15 patients withdrew before randomization. However, the CONSORT flow diagram (Fig. 1) indicates 90 patients were randomized, after which 15 withdrew (a 16.7% dropout rate), leaving 75 for the final analysis. This inconsistency obscures the potential for attrition bias.
Subgroup Analyses
The paper’s primary positive conclusion relies entirely on a subgroup analysis of the “most symptomatic” one-third of patients.
-
Credibility: This analysis was pre-specified in the methods section, which increases its credibility over a post hoc analysis. The effect was also consistent across multiple PROs (TSQ-36, GHQ-12, BDI).
-
Major Flaw: The authors explicitly state that no omnibus multiplicity correction was applied to these subanalyses. When researchers conduct many subgroup tests, the risk of finding a “significant” result purely by chance (a Type I error) increases substantially. The authors acknowledge this as a “significant limitation.” Therefore, these subgroup findings should be viewed with high skepticism and are best considered hypothesis-generating, not definitive.
Appraisal of Patient-Reported Outcomes (CONSORT-PRO)
The study’s use of PROs as pre-specified primary outcomes is a methodological strength. However, the main symptom questionnaire used, the TSQ-36, was designed by the investigators for this study and is not a formally validated instrument, though it was modeled after other questionnaires. The 16.7% dropout rate (if the flow diagram is correct) also means that PRO data is missing from a portion of the randomized cohort, which could bias the results.
Reporting Quality Assessment (CONSORT)
As required by CONSORT, the paper includes a participant flow diagram (Fig. 1) and describes the methods for randomization and blinding. However, as noted above, the paper fails a key CONSORT principle by presenting contradictory information between the text and the flow diagram regarding participant dropout. This is a critical omission that impacts the assessment of the trial’s validity.
Applicability
The study population (military health beneficiaries, stable on LT4) is generally representative of a primary care population. However, the exclusion of patients with cardiac disease or those on psychotropic medications limits generalizability to patients with these common comorbidities. The interventions (LT4, DTE, LT4+LT3) are all available in clinical practice.
🎯 Research Objective
The objective was to compare the effectiveness of LT4, DTE, and LT4+LT3 therapy in hypothyroid patients, both as a whole group and specifically within the subgroup of patients who remain most symptomatic on LT4 monotherapy.
📖 Study Design
This was a prospective, randomized, double-blind, crossover trial. 75 patients with primary hypothyroidism completed the study. Patients were randomly allocated to a sequence of three treatment arms: (1) LT4 monotherapy, (2) LT4+LT3 combination, and (3) DTE. Each treatment period lasted 22 weeks (6 weeks for titration, 16 weeks on a stable dose), after which patients “crossed over” to the next treatment arm.
👥 Setting and Participants
-
Setting: Patients were recruited from primary care clinics at Walter Reed National Military Medical Center.
-
Participants: 75 military health care beneficiaries completed the study.
-
Key Inclusion Criteria: Ages 18-65, diagnosed with primary hypothyroidism, and on a stable dose of LT4 for at least 6 months.
-
Key Exclusion Criteria: Pregnancy, cardiac disease (particularly coronary artery disease), malabsorption disorders, active cancer, uncontrolled psychosis, and use of certain medications, including psychotropic drugs.
📚 Bibliographic Data
-
Title: Comparative Effectiveness of Levothyroxine, Desiccated Thyroid Extract, and Levothyroxine+Liothyronine in Hypothyroidism
-
Authors: Mohamed K.M. Shakir, Daniel I. Brooks, Elizabeth A. McAninch, Tatiana L. Fonseca, Vinh Q. Mai, Antonio C. Bianco, and Thanh D. Hoang
-
Journal: The Journal of Clinical Endocrinology & Metabolism
-
Year: 2021
Mandatory Disclaimer: This AI-generated analysis is for informational and research purposes only and is not a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of a qualified health provider with any quest1ions you may have regarding a medical condition.
Original Article:
Full text pdf: Comparative Effectiveness of Levothyroxine, Desiccated Thyroid Extract, and Levothyroxine+Liothyronine in Hypothyroidism
