Comment:
This is a crucial systematic review that directly addresses a common clinical misconception. For years, the standard advice has been to stop fish oil before surgery due to a theoretical risk of bleeding based on its known effects on platelet aggregation.
What this review clearly demonstrates is that this theoretical risk does not translate into an actual, clinically relevant increase in perioperative bleeding for patients. The evidence presented here is strong, separating a biochemical effect from a clinical outcome.
The authors’ conclusion is well-supported: discontinuing fish oil supplements before surgery appears to be an unnecessary precaution. And we know other research shows it may be harmful.
Summary:
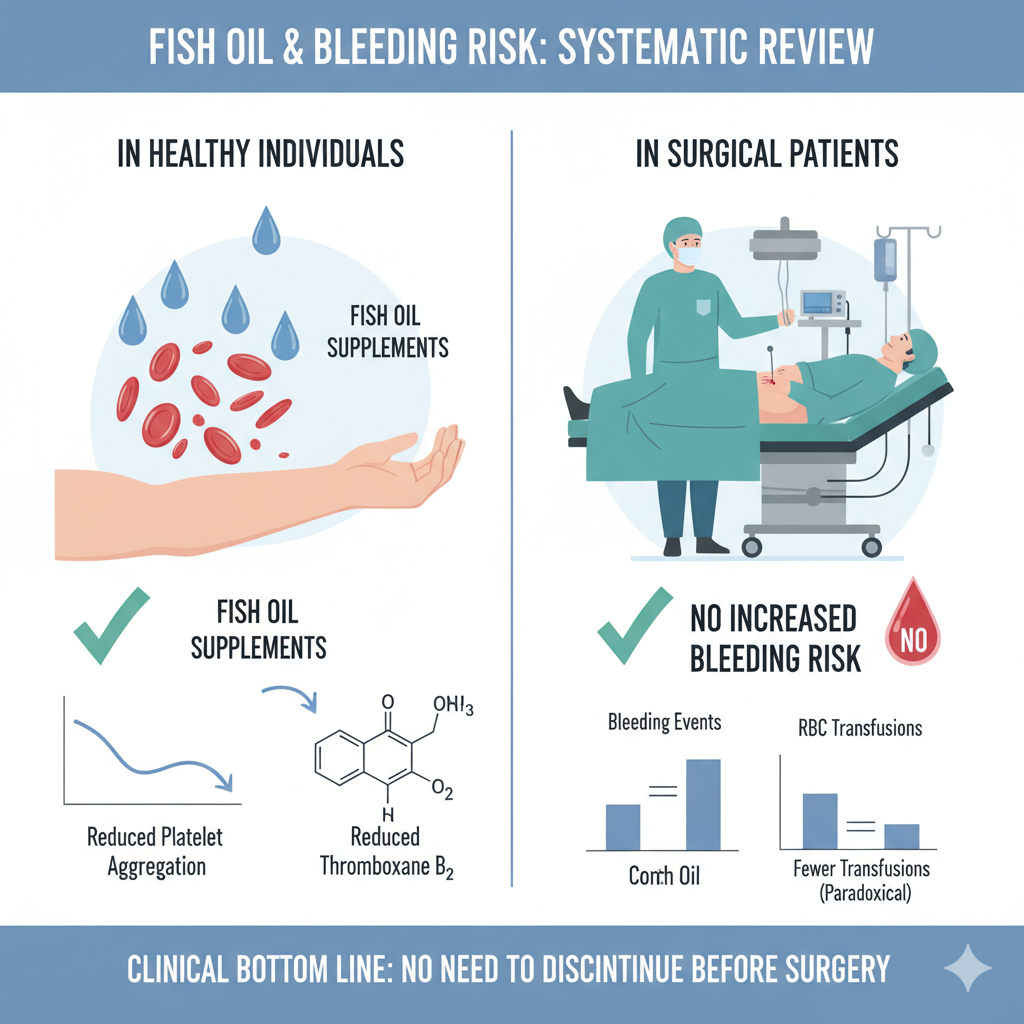
Clinical Bottom Line
This systematic review concludes that while fish oil supplements do have a measurable biochemical effect on reducing platelet aggregation in healthy individuals, this effect does not translate into an increased risk of bleeding for patients during or after surgery. Based on this evidence, the authors assert that discontinuing fish oil supplements prior to surgery or other invasive procedures is not necessary.
Results
Summary of Results
The review included 52 publications, which were separated into two groups: 32 studies on healthy subjects and 20 studies on patients undergoing surgery.
-
In Healthy Subjects: The review confirmed a known biochemical effect. The majority of studies (9 of 16) demonstrated that fish oil supplementation reduced platelet aggregation, and four out of five studies investigating thromboxane $B_2$ found its levels were reduced.
-
In Surgical Patients: This biochemical effect did not result in clinically significant bleeding. Across 16 studies that evaluated clinical bleeding in surgical patients (the majority of which were Randomized Controlled Trials), none reported an increase in intra-operative or post-operative bleeding in those exposed to fish oil.
-
Paradoxical Finding: Two large RCTs involving cardiac surgery patients paradoxically reported fewer red blood cell transfusions in the fish oil groups compared to the control groups.
-
Concomitant Antithrombotics: The review also examined literature on the combined use of fish oil and antithrombotic medications (like aspirin) and concluded that this combination does not appear to increase clinically significant bleeding.
Assertive Critical Appraisal
Risk of Bias and Certainty of Evidence
The authors did not perform a quantitative meta-analysis, so pooled statistics for the effect size or heterogeneity (like an $I^2$) are not available. The authors appropriately identify the wide variation in fish oil doses and duration of supplementation across studies as a limitation.
Reporting of Harms
A significant limitation, clearly identified by the authors, is the high potential for reporting bias. Bleeding was not the primary endpoint in most of the included surgical trials (only two, both retrospective, had it as a primary endpoint). This is a critical flaw, as bleeding events may not have been systematically registered or reported, which could lead to an underestimation of risk.
Publication Bias
The authors do not state whether they formally assessed for publication bias (e.g., using a funnel plot or Egger’s test). This is an omission, as publication bias is a common threat that could skew the results, particularly if smaller studies that did find a bleeding risk were never published.
Reporting Quality Assessment (PRISMA)
The reporting quality of this review is a strength. The authors explicitly state they followed the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines.
-
PRISMA Flow Diagram: The paper includes a clear flow diagram (Figure 1) that details the entire study selection process, showing how they arrived at the 52 included publications from the initial 3,435 records identified.
-
Search Strategy: The authors do describe their full search strategy, providing the specific search terms used for both the MEDLINE/PubMed and Embase databases, which enhances the transparency and reproducibility of their review.
Research Objective
To systematically review the effect of fish oil supplements (Intervention) on haemostasis (e.g., platelet aggregation) and clinical bleeding risk (Outcomes) in healthy subjects and in patients undergoing surgery or invasive procedures (Populations), compared to control groups or baseline measures (Comparator).
Study Design
This is a systematic review conducted according to PRISMA guidelines. The authors searched the MEDLINE/PubMed and Embase databases for original research published since 1960. They included studies on healthy subjects (requiring a minimum of 20 subjects) and patients undergoing surgery (with no minimum number) who received fish oil supplements and had data on haemostasis or clinical bleeding. Reviews, animal studies, and studies on fish diet (as opposed to supplements) were excluded.
Setting and Participants
The review included a total of 52 publications for the final synthesis. These were categorized into 32 publications involving healthy subjects and 20 publications involving patients undergoing surgery or invasive procedures1.
Bibliographic Data
-
Title: No impact of fish oil supplements on bleeding risk: a systematic review
-
Authors: Katrine Munk Begtrup, Andreas Engel Krag & Anne-Mette Hvas
-
Journal: Danish Medical Journal (Dan Med J)
-
Year: 2017
-
DOI: Not provided in the document.
Original Article:
Full text pdf: No impact of fish oil supplements on bleeding risk a systematic review
Open Access Creative Commons License
Opdateret 1. december 2023
Open Access Creative Commons License: CC BY-NC-ND 4.0.
