Comment:
Although there are some potential benefits to hormone replacement therapy, there are significant risks associated as well. This study is another one that shows significant cardiovascular harm, with increases in stroke and thromboembolism.`
Summary:
Clinical Bottom Line
This high-certainty meta-analysis demonstrates that for the general postmenopausal population, menopause hormone therapy (MHT) is associated with significant cardiovascular harm. Specifically, MHT increases the risk of stroke by 23% and venous thromboembolism by 86%. Importantly, this analysis found no corresponding benefit in reducing all-cause death or major cardiovascular events, indicating a net negative risk-benefit balance for cardiovascular outcomes.
Harms and Negative Outcomes
Summary of Key Risks
The pooled data from 33 randomized controlled trials involving 44,639 women revealed a statistically significant increase in serious thromboembolic events with MHT compared to placebo:
-
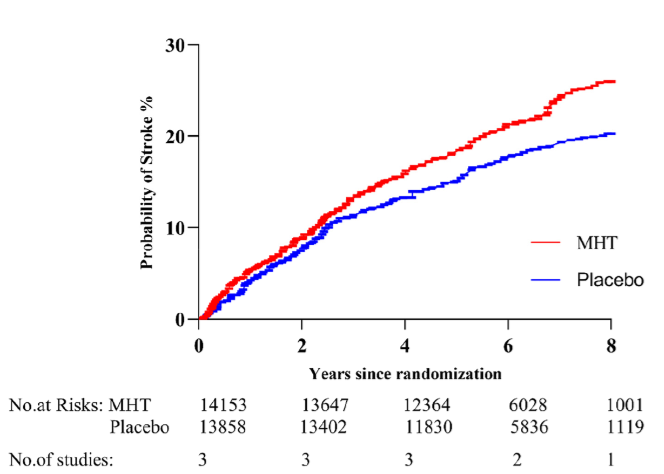
Stroke: The risk of stroke was significantly increased in women taking MHT (Risk Ratio [RR] = 1.23; 95% CI 1.08 to 1.41). A Risk Ratio (RR) of 1.23 means that the risk of experiencing a stroke was 23% higher in the MHT group than in the placebo group.
-
Venous Thromboembolism (VTE): The risk of VTE was markedly increased (RR = 1.86; 95% CI 1.39 to 2.50), indicating that women on MHT had an 86% higher risk of VTE compared to those on placebo.
Absence of Cardiovascular Benefit
The meta-analysis also confirmed that MHT does not confer a protective effect on major cardiovascular endpoints:
-
All-Cause Death: No significant difference was found between MHT and placebo (RR = 0.96; 95% CI 0.85 to 1.09).
-
Cardiovascular Events: No significant difference was observed (RR = 0.97; 95% CI 0.82 to 1.14).
Assertive Critical Appraisal
Certainty of Evidence (GRADE Framework)
The evidence for the key harmful outcomes—stroke and venous thromboembolism—was rated as High certainty by the authors using the GRADE framework. This high level of certainty strengthens the conclusion that these risks are real and clinically significant.
Risk of Bias in Included Studies
While the overall risk of bias across the 33 included studies was assessed as low, the authors noted that four trials had a high risk of bias. These trials had flaws in randomization, blinding, or handling of outcome data. Although this is a minority of the included evidence, it represents a potential weakness that could introduce some uncertainty into the pooled estimates.
Research Objective
To evaluate the cardiovascular benefits and risks (all-cause death, cardiovascular events, stroke, venous thromboembolism, FMD, and NMD) of menopause hormone therapy (MHT) compared to placebo or no treatment in postmenopausal women.
Study Design
This is a systematic review and meta-analysis of Randomized Controlled Trials (RCTs). The search included the EMBASE, MEDLINE, and CENTRAL databases for RCTs published between 1975 and July 2022. The review process followed standard Cochrane and GRADE guidelines.
Setting and Participants
The analysis included 33 RCTs with a total of 44,639 postmenopausal women. The mean age of participants was 60.3 years.
Bibliographic Data
-
Title: The benefits and risks of menopause hormone therapy for the cardiovascular system in postmenopausal women: a systematic review and meta-analysis
-
Authors: Gu Y, Han F, Xue M, Wang M, Huang Y
-
Journal: BMC Women’s Health
-
Year: 2024
Mandatory Disclaimer: This AI-generated analysis is for informational and research purposes only and is not a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of a qualified health provider with any questions you may have regarding a medical condition.
Original Article:
Open Access
To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
