Comment:
This is a fascinating retrospective study that directly challenges a core assumption in conventional endocrinology. The prevailing dogma is that synthetic levothyroxine provides superior TSH stability, with desiccated thyroid extract (DTE) often dismissed as an inconsistent or unstable option.
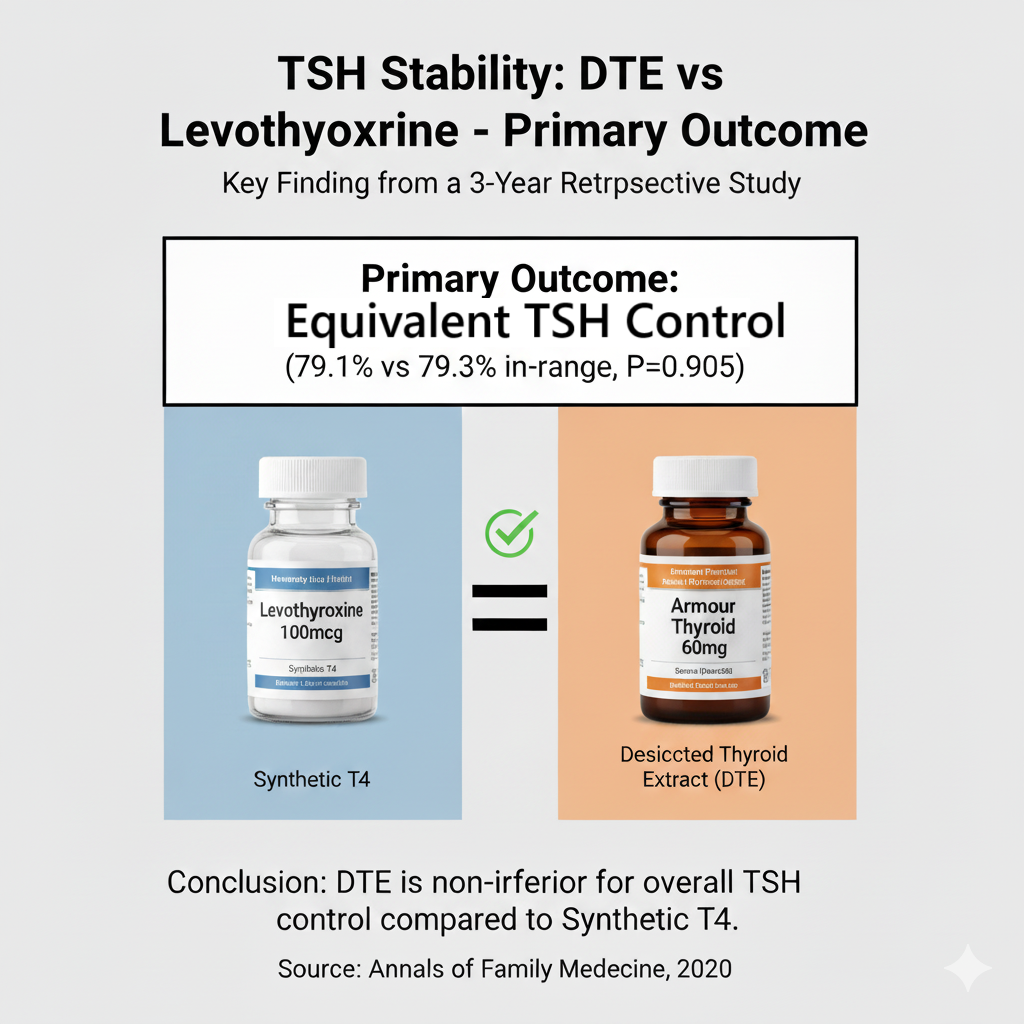
However, this real-world data demonstrates that for the primary clinical goal—keeping a patient’s TSH within the normal range over a 3-year period—DTE was statistically equivalent to synthetic T4. This shows that the intrenched view that DTE’s is inherently unstable is not an issue in real-world clinical practice.
Summary:
Clinical Bottom Line
This retrospective cohort study found no significant difference in the primary measure of long-term TSH control—the percent of TSH values within the normal range —over a 3-year period for patients taking synthetic levothyroxine versus those taking desiccated thyroid products.
However, the study did find that patients on synthetic levothyroxine had statistically less visit-to-visit variability in their TSH levels. While this challenges the common guideline assumption that synthetic products provide superior overall stability in real-world practice, the lower variability may be clinically meaningful for providers aiming for a very specific TSH target. As an observational study, these findings show an association and cannot prove causation; they are subject to significant limitations, most notably the inability to account for patient adherence.
Results in Context
-
Main Results: The primary outcome, the mean percent of TSH values in the euthyroid range (0.320-5.500 uIU/mL), was nearly identical between the two groups.
-
Synthetic Levothyroxine: 79.1% of TSH values were in-range.
-
Desiccated Thyroid: 79.3% of TSH values were in-range.
-
This difference was not statistically significant (P=0.905).
-
-
Key Secondary Results:
-
Visit-to-Visit TSH Variability: Patients on synthetic levothyroxine had significantly less variability between TSH measurements (1.25 vs 1.44, P=0.015).
-
Patients Always in Range: There was no difference in the percentage of patients who had 100% of their TSH values in-range during the 3-year study period (59.8% for synthetic vs. 60.0% for desiccated, P=0.951).
-
-
Participants: The study included 870 total patients (435 in each group). The cohorts were matched 1:1 and were similar in age (mean ~63 years), sex (~90% female), and race/ethnicity.
Assertive Critical Appraisal
-
Limitations & Bias (STROBE Framework): The study’s primary weakness is its retrospective design. This design is vulnerable to unmeasured confounding variables, meaning factors other than the medication type could be responsible for the results. The authors appropriately acknowledge this limitation.
-
Reporting Quality (STROBE/RECORD):
-
Confounding: While the authors used matching (age, sex, race) and statistical adjustment for some comorbidities (like diabetes and hypertension) to address confounding, they explicitly note several critical unmeasured confounders. Most importantly, they could not account for patient adherence, differences in prescriber practices, or the use of concurrent medications that might affect TSH levels. Poor adherence in one group could easily mask a true difference in drug stability.
-
Baseline Differences: Despite matching, the groups had statistically significant differences at baseline. The desiccated thyroid group had a lower average BMI, lower HgbA1c, and a lower (though still normal) baseline TSH. This suggests that the patients who received desiccated thyroid were meaningfully different from those who received levothyroxine, perhaps reflecting different patient preferences or prescriber habits that could bias the results.
-
Data Source (RWE): The study used “real-world data” from an electronic medical record (EMR) system. While this is good for assessing real-world applicability, EMR data is notoriously poor for capturing patient adherence, which is a key variable in this analysis.
-
-
Applicability: The findings are from a large, integrated US health system (Kaiser Permanente Colorado) and represent a typical outpatient population being treated for hypothyroidism. The results are directly relevant to general clinicians managing this common condition.
Research Objective
The study’s purpose was to evaluate and compare the long-term stability of TSH levels in patients with hypothyroidism who were prescribed either synthetic levothyroxine or desiccated thyroid products.
Study Design
This was a retrospective matched-cohort study. Patients were identified from electronic medical, pharmacy, and laboratory records. Patients on synthetic levothyroxine were matched 1:1 to patients on desiccated thyroid based on age, sex, and race/ethnicity. Data was collected for a 3-year follow-up period after an “index date,” which was defined as the second dispensing of the same thyroid product at least one year after the first (to ensure chronic use).
Setting and Participants
-
Setting: The study was conducted at Kaiser Permanente Colorado (KPCO).
-
Participants: The study included 870 adult patients (≥18 years) who were receiving chronic treatment for hypothyroidism with either synthetic levothyroxine or desiccated thyroid between January 1, 2005, and December 31, 2015.
-
Exclusions: Patients were excluded if they had conditions like thyroid cancer, Graves’ disease, Hashimoto’s thyroiditis, panhypopituitarism, or were pregnant. Patients using more than one type of thyroid agent were also excluded.
📚 Bibliographic Data
-
Title: Thyroid Stimulating Hormone Stability in Patients Prescribed Synthetic or Desiccated Thyroid Products: A Retrospective Study
-
Authors: Rolake Kuye, Catherine Riggs, Jordan King, Rachel Heilmann, Deanna Kurz, Jessica Milchak
-
Journal: Annals of Family Medicine
-
Year: 2020
This AI-generated analysis is for informational and research purposes only and is not a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of a qualified health provider with any questions y9ou may have regarding a medical condition.
Original Article:
Full text pdf: hyperlink here
